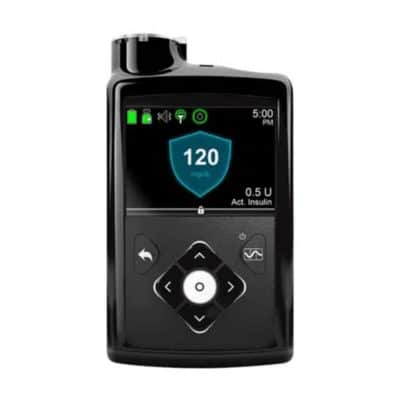
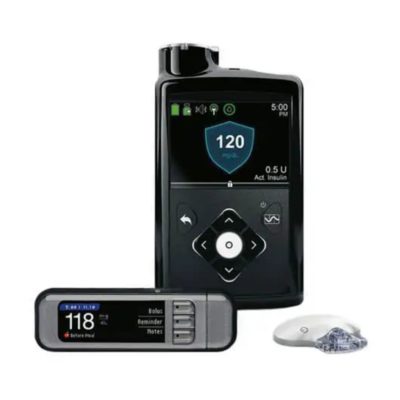
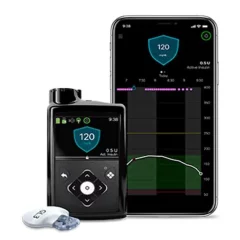
MiniMed™ 630G Insulin Pump System
Simplify insulin delivery with the MiniMed 630G Insulin Pump System. Medtronic’s MiniMed pumps are the only devices on the market with SmartGuard, a technology made to step in when your glucose levels go under a predetermined level.* You’ll also get predictive alerts of high or low glucose trends up to half an hour in advance.
Thanks to these features, MiniMed 630G System users may find it easier to stay in range† and reach their target A1C.‡
Please call our Customer Care Team at 1-866-422-4866 or fill out the form below to see if you qualify for an insulin pump system with $0 out-of-pocket costs.**
- SmartGuard technology can respond to low glucose levels*
- Color screen with automatic brightness adjustments
- Simple for anyone to use
- Guardian Sensor 3 adds optional CGM
- Sensor is wearable for up to seven days
The MiniMed 630G Insulin Pump System can automatically deliver insulin to people with type 1 or type 2 diabetes. Along with this, it can offer CGM capabilities when paired with other products from Medtronic.
How Does The MiniMed 630G System’s SmartGuard Work?
The SmartGuard system takes action when your glucose levels dip below a certain point. If you don’t respond to alerts, the system can pause insulin delivery for up to two hours.¶
Is The MiniMed 630G System A CGM?
The MiniMed 630G System does not have integrated CGM capabilities. However, it can work with the Guardian Sensor 3 and Guardian Link 3 Transmitter to provide CGM features to its users.
Does The MiniMed 630G System Have Health Benefits?
According to Medtronic, this system can lower users’ risk of complications related to kidney damage, nerve damage, cardiovascular damage, and eye damage.#
How Often Does The MiniMed 630G System Update Glucose Readings?
When used with the Guardian Sensor 3 and Guardian Link 3 Transmitter, the MiniMed 630G System can deliver updated glucose readings every five minutes.
Administration of continuous subcutaneous insulin for the treatment of diabetes mellitus (Refer to the ICD-10 code list in the LCD-related Policy Akicle for applicable diagnoses.) if criterion A or B is met and if criterion C or D is met:
-
C-peptide testing requirement – must meet criterion 1 or 2 and criterion 3:
-
C-peptide level is less than or equal to 110 percent of the lower limit of normal of the Jaborator’s measurement method.
-
For beneficiaries with renal insufficiency and a creatinine clearance (actual or calculated from age, weight, and serum creatinine) less than or equal to 50 ml/minute, a fasting C-peptide level is less than or equal to 200 per cent of the lower limit of normal of the laboratory’s measurement method.
-
A fasting blood sugar obtained at the same time as the C-peptide level is less than or equal to 225 mg/dI.
-
-
Beta cell autoantibody test is positive.
-
The beneficiary has completed a comprehensive diabetes education program, has been on a program of multiple daily injections of insulin (i.e., at least 3 injections per day) with frequent self-adjustments of insulin dose for at least 6 months prior to initiation of the insulin pump, and has documented frequency of glucose self-testing an average of at least 4 times per day during the 2 months prior to initiation of the insulin pump, and meets one or more of the following criteria (1-5) while on the multiple injection regimen:
-
Glycosylated hemoglobin level (HbA1C) greater than 7 percent
-
History of recurring hypoglycemia
-
Wide fluctuations in blood glucose before mealtime
-
Dawn phenomenon with fasting blood sugars frequently exceeding 200 mg/dL
-
History of severe glycemic excursions
-
-
The beneficiary has been on an external insulin infusion pump prior to enrollment in Medicare and has documented frequency of glucose self-testing an average of at least 4 times per day during the month prior to Medicare enrollment.
If criterion A or B is not met, the pump and related accessories, supplies, and insulin will be denied as not reasonable and necessary. If criterion C or D is not met, the pump and related accessories, supplies, and insulin will be denied as not reasonable and necessary.
Continued coverage of an external insulin pump and supplies requires that the beneficiary be seen and evaluated by the treating practitioner at least every 3 months. In addition, the external insulin infuser
*CGM uses a special sensor to measure sugar levels just below the skin known as interstitial fluid. These sensor glucose (SG) values are different from blood glucose (BG) measurements using a BG meter. Sensor glucose values should not be used to make treatment decisions. Patients should always do a BG fingerstick before they make treatment decisions.
†Battelino T, Conget I, Olsen B, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomized controlled trial. Diabetologia. 2012;55:3155–3162.
‡Doyle EA, Weinzimer SA, Steffen AT, Ahern JAH, Vincent M, Tamborlane WV. A randomized prospective trial comparing the efficacy of insulin pump therapy with multiple daily injections using insulin glargine. Diabetes Care. 2004;27( 7 ):1554–1558.
§At the time of manufacture and when the reservoir and tubing are properly inserted, your pump is waterproof. It is protected against the effects of being underwater to a depth of up to 12 feet (3.6 meters) for up to 24 hours.
¶Refers to suspend on low. WARNING: Do not use the Suspend on low feature to prevent or treat low glucose. Always confirm your sensor glucose reading using your BG meter, and follow the instructions of your healthcare professional to treat low glucose. Using Suspend on low alone to prevent or treat low glucose may result in prolonged hypoglycemia.
#The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986.
**For qualified persons with applicable in-network insurance and subject to CGM coverage requirements. Deductibles, co-pays, and other conditions apply. Speak to an ADS representative and your insurance company to confirm your coverage and costs.